Development
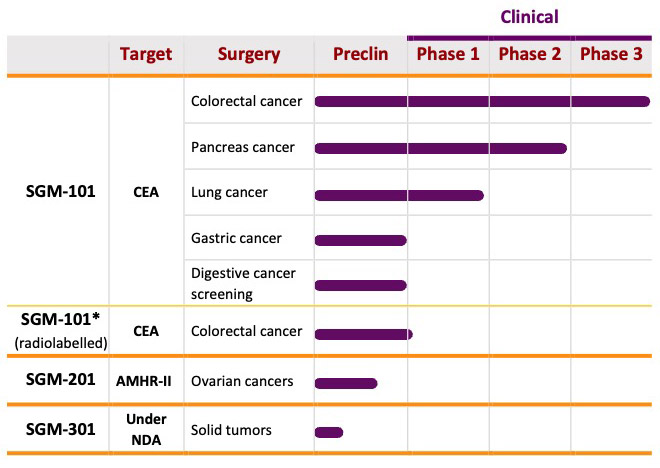
Pipeline
SurgiMab is currently working on the development of two products, with ongoing external collaborations on additional candidates. SGM-101, currently in Phase III for the delineation of primary and recurrent tumor and metastases in patients undergoing surgery for colorectal cancer, is a tumor-specific antibody conjugated to a near-infrared fluorochrome. It selectively binds a specific marker overexpressed in gastrointestinal and other tumors. SGM-201 is a preclinical stage product based on the same technique that targets ovarian cancer. Both allow surgeons to visualize tumors in real-time with a near-infrared camera, helping them to perform more radical cytoreductive surgery, thus automatically improving surgical outcomes.
“Fluorescence-guided surgery (FGS) is an exciting new approach that allows the surgeon in real-time to differentiate between tumor tissue and healthy tissue, enabling detection of even small metastatic nodules that are invisible to the eye. With more than 400,000 new cases of CRC diagnosed every year in Europe, new approaches that can facilitate the detection of malignant tissue and potentially improve patient outcomes are greatly needed. We look forward to further evaluating the potential of SGM-101 …”
Dr. Alexander Vahrmeijer, coordinating investigator of the Phase II and III studies. Leiden University Medical Center. Leiden NL
“Sometimes we detect extra lesions, deeper infiltration. We require less frozen sections. Sometimes even we do a lesser procedure than we had anticipated. But even in normal cases, without changes in the surgical plan it helps to make the surgeon more confident about what he is doing …”
Dr. Harm Rutten, participating investigator of the Phases II and III studies. Catharina Ziekenhuis, Eindhoven NL.
Clinical Studies
Phase III studies
Colorectal Cancer
The randomized Phase 3 trial, designed following discussions with the FDA and other regulators, aims to enroll 300 CRC patients in ten clinical centers in Europe and US, and will assess the clinical benefit of using FGS with SGM-101 as the intraoperative imaging agent to identify cancer lesions during the surgical procedure. Patients receive 10mg of SGM-101 four days prior to the scheduled CRC surgical procedure. Preliminary clinical data of the Phase 3 trial is expected in 2023. READ MORE ABOUT THIS CLINICAL TRIAL Another Phase 3 trial is currently ongoing in the Netherlands. It is a national multicenter, open label clinical trial on the performance of SGM-101for the delineation of locally advanced and recurrent rectal cancer. Patients will be followed for a total duration of two years postoperatively. READ MORE ABOUT THIS CLINICAL TRIAL
Phase I/II Study
Preliminary results for the Phase I/II trial, regarding 26 patients (nine from the dose-escalating cohort and 17 in the expansion cohort, presenting with recurrent or peritoneal metastases of colorectal cancer), were published in the Lancet Gastroenterology & Hepatology (Boogerd L.S.F. et al. 2018). It was shown that “in the expansion cohort, 19 (43%) of 43 lesions were detected using fluorescence imaging and were not clinically suspected before fluorescent detection, which changed the treatment strategy in six (35%) of 17 patients.“
Near Infrared Surgery (NIRS). Omroep Brabant/Catharina Hospital Eindhoven
Near-infrared fluorescence detection of retroperitoneal lymph node metastases. Supplementary video from the article by Boogerd L.S.F. et al., 2018 The Lancet Gastroenterology & Hepatology 3(3): 181-191. DOI: https://doi.org/10.1016/S2468-1253(17)30395-3
SGM-101 has been tested in an phase I/II trial in the Netherlands, with patients operated at Leiden University Medical Center, Catharina Ziekenhuis (Eindhoven) and Erasmus MC (Rotterdam). 75 patients undergoing surgery for colorectal cancer or pancreatic cancer were included in the trial, that is now complete.
- Title : Study on the detection of cancer during surgery for rectal cancer or pancreatic cancer with the fluorescent agent SGM-101.
- Primary outcome : Safety and tolerability
- Secondary outcome : Performance of SGM-101
First-in-human study
80% of cancer patients are treated with surgery as first intention. Up to 40% relapse within 2 years because surgeons left some tumor in place, due to their inability to clearly distinguish tumor from normal tissue with only vision or palpation. SGM-101 safety was demonstrated in a first-in-human study in Montpellier Cancer Institute.
- Title : Phase I Study assessing safety of SGM-101, a fluorochrome-labeled anti-CEA agent monoclonal antibody … in patients with peritoneal carcinomatosis from CEA-expressing digestive cancer.
- Primary outcome : Maximum Tolerated Dose, Recommended phase II dose
- Secondary outcome : Safety profile, Pharmacokinetic profile
Publications
2023
- Meijer RPJ, Galema HA, Faber RA, Bijlstra OD, Maat APWM, Cailler F, Braun J, Keereweer S, Hilling DE, Burggraaf J, Vahrmeijer AL, Hutteman M; SGM-CLM study group. Intraoperative molecular imaging of colorectal lung metastases with SGM-101: a feasibility study. Eur J Nucl Med Mol Imaging. 2023 Aug 8. doi: 10.1007/s00259-023-06365-3. Epub ahead of print. PMID: 37552367. Click here to view the pdf.
- Azari F, Meijer RPJ, Kennedy GT, Hanna A, Chang A, Nadeem B, Din A, Pèlegrin A, Framery B, Cailler F, Sullivan NT, Kucharczuk J, Martin LW, Vahrmeijer AL, Singhal S. Carcinoembryonic Antigen-Related Cell Adhesion Molecule Type 5 Receptor-Targeted Fluorescent Intraoperative Molecular Imaging Tracer for Lung Cancer: A Nonrandomized Controlled Trial. JAMA Netw Open. 2023 Jan 3;6(1):e2252885. Click here to view the pdf.
2022
- Nishino H, Turner MA, Amirfakhri S, Hollandsworth HM, Lwin TM, Hosseini M, Framery B, Cailler F, Pèlegrin A, Hoffman RM, Bouvet M. Proof of concept of improved fluorescence-guided surgery of colon cancer liver metastasis using color-coded imaging of a tumor-labeling fluorescent antibody and indocyanine green restricted to the adjacent liver segment. Surgery. 2022 Oct;172(4):1156-1163. Click here to view the pdf.
- Azari F, Kennedy GT, Chang A, Bernstein E, Nadeem B, Pèlegrin A, Cailler F, Sullivan NT, Kucharczuk J, Singhal S. Glycoprotein Receptor CEACAM5-Targeted Intraoperative Molecular Imaging Tracer in NSCLC. Ann Thorac Surg. 2022 May 26:S0003-4975(22)00731-7. Click here to view the pdf.
- Galema HA, Meijer RPJ, Lauwerends LJ, Verhoef C, Burggraaf J, Vahrmeijer AL, Hutteman M, Keereweer S, Hilling DE. Fluorescence-guided surgery in colorectal cancer; A review on clinical results and future perspectives. Eur J Surg Oncol. 2022 Apr;48(4):810-821. Click here to view the pdf.
2021
- Azari F, Kennedy G, Bernstein E, Hadjipanayis C, Vahrmeijer A, Smith B, Rosenthal E, Sumer B, Tian J, Henderson E, Lee A, Nguyen Q, Gibbs S, Pogue B, Orringer D, Charalampaki C, Martin L, Tanyi J, Lee M, Lee JY, Singhal S. Intraoperative molecular imaging clinical trials: a review of 2020 conference proceedings. J Biomed Opt. 2021 May;26(5):050901. Click here to view the pdf.
- Meijer RPJ, de Valk KS, Deken MM, Boogerd LSF, Hoogstins CES, Bhairosingh SS, Swijnenburg RJ, Bonsing BA, Framery B, Fariña Sarasqueta A, Putter H, Hilling DE, Burggraaf J, Cailler F, Mieog JSD, Vahrmeijer AL. Intraoperative detection of colorectal and pancreatic liver metastases using SGM-101, a fluorescent antibody targeting CEA. Eur J Surg Oncol. 2021 Mar;47(3 Pt B):667-673. Click here to view the pdf.
- de Valk KS, Deken MM, Schaap DP, Meijer RP, Boogerd LS, Hoogstins CE, van der Valk MJ, Kamerling IM, Bhairosingh SS, Framery B, Hilling DE, Peeters KC, Holman FA, Kusters M, Rutten HJ, Cailler F, Burggraaf J, Vahrmeijer AL. (2021) Dose-Finding Study of a CEA-Targeting Agent, SGM-101, for Intraoperative Fluorescence Imaging of Colorectal Cancer. Ann Surg Oncol. 28(3):1832-1844. Click here to view the pdf.
2020
- Schaap D.P., de Valk K. S., Deken M. M., Meijer R. P. J., Burggraaf, J., Vahrmeijer A. L., and Kusters M., on behalf of the SGM‐101 study group. (2020). Carcinoembryonic antigen‐specific, fluorescent image‐guided cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer. Br J Surg. 107(4): 334–337. Click here to view the pdf.
- de Gooyer JM, Elekonawo FMK, Bos DL, van der Post RS, Pèlegrin A, Framery B, Cailler F, Vahrmeijer AL, de Wilt JHW, Rijpkema M. Multimodal CEA-Targeted Image-Guided Colorectal Cancer Surgery using 111In-Labeled SGM-101. Clin Cancer Res. 2020 Nov 15;26(22):5934-5942. Click here to view the pdf.
2019
- Pèlegrin A., Gutowski M. and Cailler F. (2019) Antibodies, tools of choice for fluorescence-guided surgery. Med Sci (Paris) 35(12):1066-1071. Click here to view the pdf (French)
- Framery B., Gutowski M., Dumas K., Evrard A., Muller N., Dubois V., Quinonero J., Scherninski F., Pèlegrin A. and Cailler F. (2019). Toxicity and pharmacokinetic profile of SGM-101, a fluorescent anti-CEA chimeric antibody for fluorescence imaging of tumors in patients. Toxicol Rep. 6:409-415. Click here to view the pdf.
2018
- Hoogstins C.E.S., Boogerd L.S.F., Mulder B.G.S., Mieog J.S.D., Swijnenburg R.J., van de Velde C.J.H., Farina-Sarasqueta A., Bonsing B.A., Framery B., Pèlegrin A., Gutowski M., Cailler F., Burggraaf J. and Vahrmeijer A.L. (2018). Image-Guided Surgery in Patients With Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann Surg Oncol 25(11):3350-3357. Click here to view the pdf.
- Boogerd L.S.F., Hoogstins C.E.S., Schaap D.P., Kusters M., Handgraaf J.J.M., van der Valk M.J.M., Holman F.A., Peeters K.C.M.J., Mieog J.S.D., van de Velde C.J.H., Farina-Sarasqueta A., van Lijnschoten I., Framery B., Pèlegrin A., Gutowski M., Nienhuijs S.W., de Hingh I.H.J.T., Nieuwenhuijzen G.A.P., Rutten A.J.T., Cailler F., Burggraaf J. and Vahrmeijer A.L. (2018). Safety and Effectiveness of SGM-101, a Fluorescent Antibody Targeting Carcinoembryonic Antigen, for Intraoperative Detection of Colorectal Cancer: A Dose-Escalation Pilot Study. Lancet Gastroenterol Hepatol. 3(3):181-191. Click here to get to the article.
2017
- Gutowski, M. Framery, B., Boonstra M.C., Garambois, V., Quenet F., Dumas K., Scherninski F., Cailler F., Vahrmeijer A.L. and Pèlegrin A. (2017). SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surgical Oncology 26: 153-162. Click Click here to get to the article.

